Author:
V Ong1, G Hough1, S Flanagan1, K Bartizal1, A Sattar2, A Sharp2, P Thommes2, T Murphy3
Author address:
1 Drug Development, Cidara Therapeutics, San Diego, CA, USA;
2 Pharmacology, Evotec, Manchester, UK;
3 Pharmacology, NeoSome Life Sciences, Lexington, MA, USA.
Full conference title:
The 8th Advances Against Aspergillus, Lisbon Conference Center, Lisbon, Portugal
Date: 6 February 2018
Abstract:
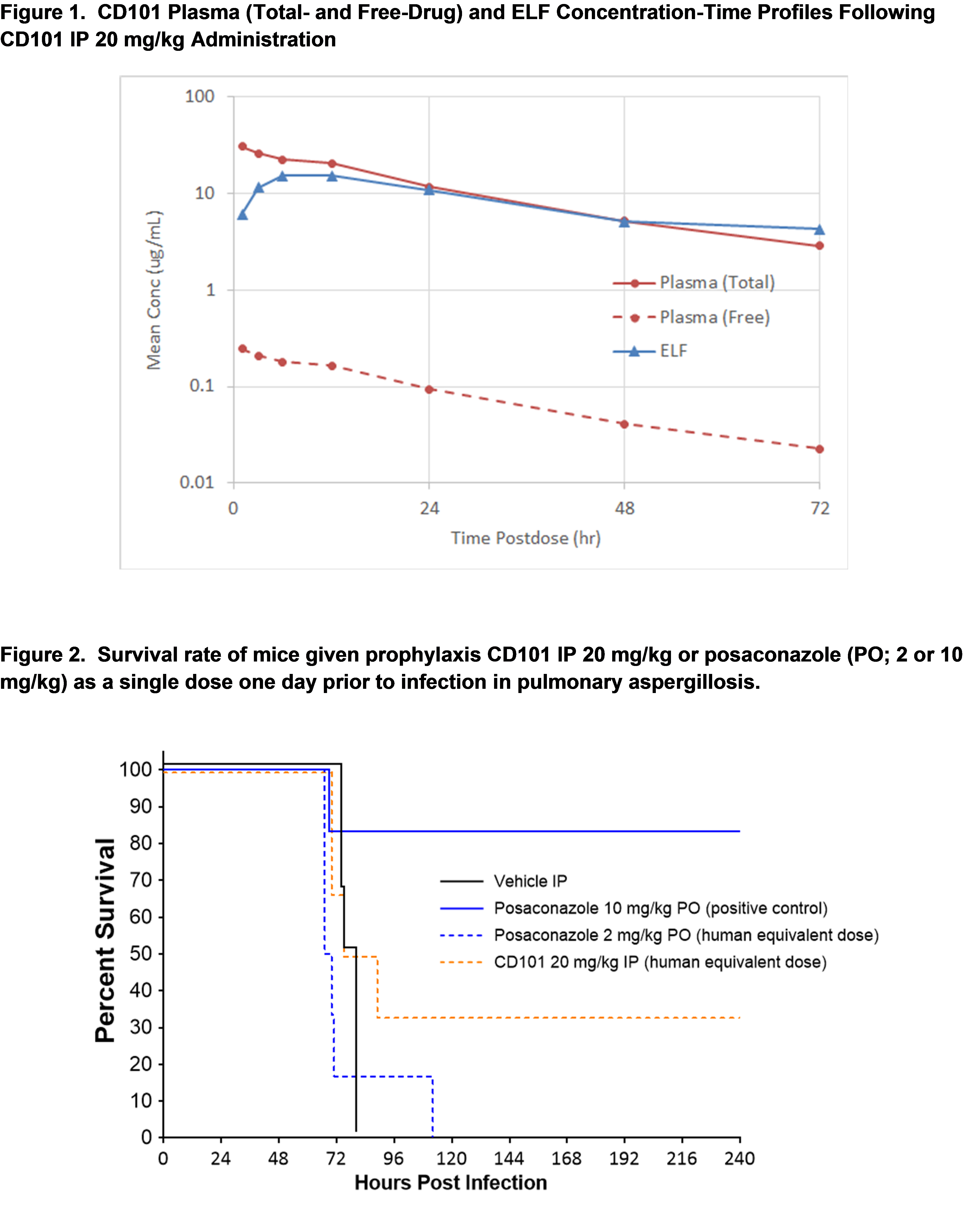
Purpose: CD101 has previously demonstrated robust efficacy as treatment and prophylaxis in mouse antifungal models of aspergillosis. The distribution of CD101 into lung ELF was studied to further substantiate this observed efficacy.
Methods: CD101 (20 mg/kg) was administered IP to 24 ICR mice. At pre-dose, 1, 3, 6, 12, 24, 48, and 72 hours post-dose, 3 mice/timepoint were euthanized for plasma and bronchoalveolar lavage fluid (BALF) collection with 2x 0.5 mL flushes of saline. Urea levels for plasma/BALF normalization for lung ELF volume calculation were quantified using a spectrophotometry-based assay. CD101 concentrations in plasma/BALF samples were measured by LC-MS/MS.
Disseminated aspergillosis: ICR mice (6/grp) were made neutropenic by cyclophosphamide on days -3 (270 mg/kg), +1 and +4 (90 mg/kg). IV challenge with A. fumigatus ATCC 13073 (104 CFU/mouse) was initiated on day 0 and CD101 treatment was administered (2h post-dose) as a single dose (2 mg/kg IV and IP) or daily (0.5 mg/kg BID) dosing. Survival was monitored for ≥10 days. The same model was used for prophylaxis except CD101 was dosed on days -1, -3 or -5.
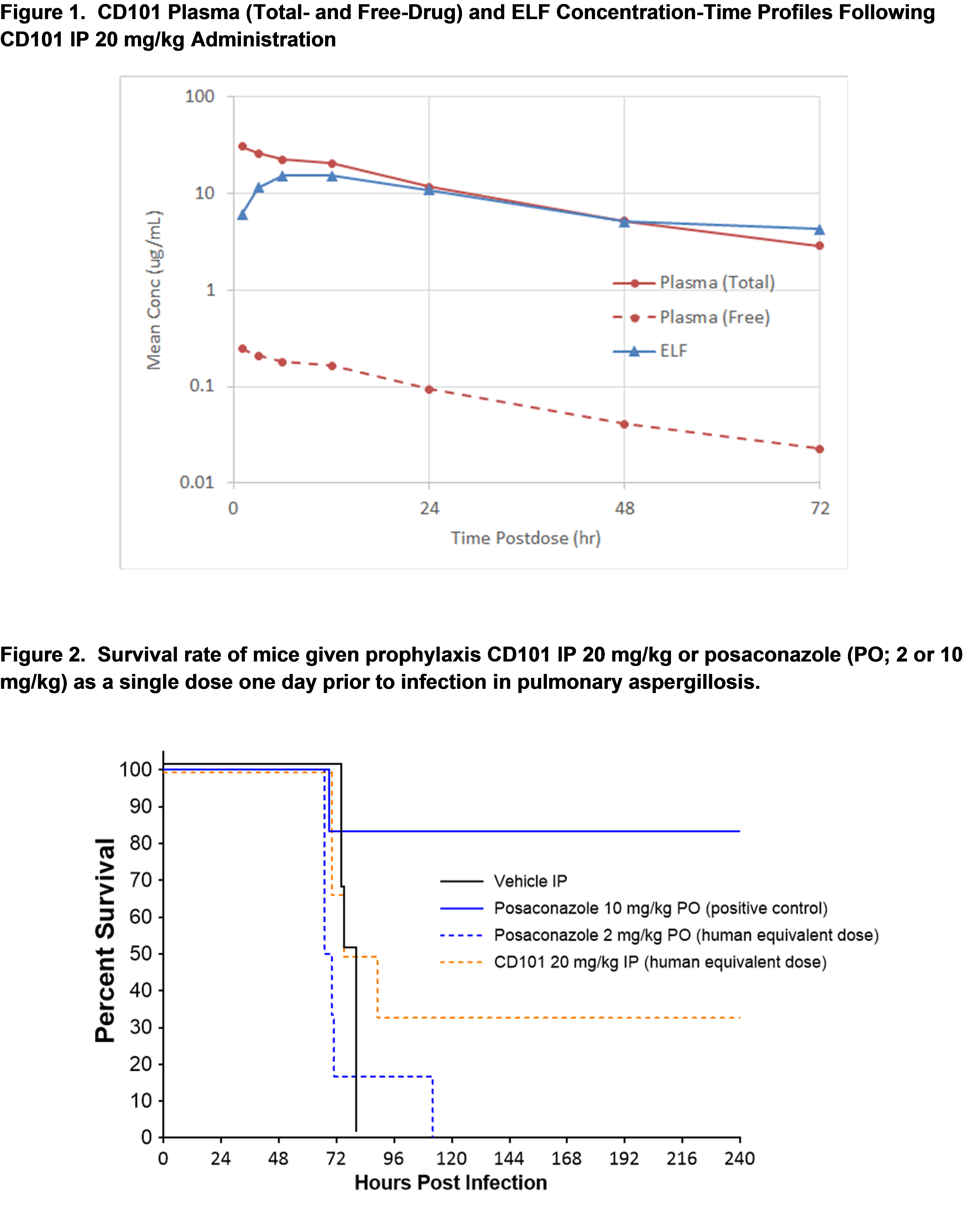
Pulmonary aspergillosis: ICR mice (10/grp) were made neutropenic by cyclophosphamide on day -4 (150 mg/kg), and cyclophosphamide/cortisone was given on day -1 (150/175 mg/kg). Intranasal challenge with A. fumigatus AF293 (105 CFU/mouse) was initiated on day 0 and prophylaxis with CD101 as a single dose (IP; 5, 10, 20 mg/kg) or posaconazole (PO; 2 and 10 mg/kg) was started 1 day prior to infection. Survival was monitored for 10 days.
Results: As lung ELF levels are free-drug concentrations while plasma concentrations represent total-drug (free plus protein-bound), plasma concentrations were also corrected for protein binding (99.2%). CD101 ELF concentrations reached a maximum by 4h and were comparable between plasma and ELF beyond 24h post-dose in total-drug concentrations but much higher based on free-drug (Figure 1). The ELF/Plasma AUClast ratio was 0.80 for total-drug and 100 for free-drug exposures, respectively.
For treatment of disseminated aspergillosis, CD101 by IV/IP at 0.2, 1, or 5 mg/kg BID x 5d showed a significant increase in survival compared to vehicle. Survival was comparable with either a single 2 mg/kg dose or 0.2 mg/kg BID x 5d. For prophylaxis, a single 5 mg/kg dose given up to 5 days prior to infection showed improved survival depending on day given. Doses ≥10 mg/kg showed 100% survival.
In the more challenging pulmonary aspergillosis model, dose-dependent increase in survival was observed from a single prophylaxis CD101 dose. The human (400 mg) AUC equivalent of 20 mg/kg in mice showed an increase in survival relative to control. Further comparison with posaconazole at the human AUC equivalent dose of 2 mg/kg (Figure 2) suggests an advantage for CD101 with 30% survival rate compared to no survivors for posaconazole. Only posaconazole at 10 mg/kg (5x higher than human AUC) showed a statistically-significant increase in survival rate relative to control.
Conclusion: With a long plasma half-life in human (133 h) and extensive lung ELF exposures, CD101 could be a viable candidate for outpatient echinocandin treatment and prophylaxis.
Abstract Number: 142
Conference Year: 2018
Link to conference website: IDWeek 2016
Tables:
 ,
, 
Link Conference abstract:
Conference abstracts, posters & presentations
-
Title
Author
Year
Number
Poster
-
v
D Luptáková1*, RH Patil1,2, R Dobiáš3,4, DA Stevens5,6, T Pluháček1,2, A Palyzová1, A Škríba1, V Havlíček1,2
2022
23
-
v
Y Bahri*, S Ben.Belgacem, Y Maatouk, A Ben.Salah, M Mastouri
2022
22
-
v
Y Bahri*, S Ben.Belgacem, Y Maatouk, A Ben.Salah, M Mastouri
2022
21
-
v
A Knaz1, A Omrane1, H Chouaieb2, M Chatti2, R Mrassi2, S Ismail2, M Ben Seif2, W Benzarti1, MA Ayed1, A Abdelghani1, A Hayouni1, S Aissa1, I Gargouri1, A Fathallah2*
2022
20
-
v
A Azzabi1, O Mahfoudh1, N Ben Aicha1, W Sahtout1, A Ayadi2, Z Lajmi3, M Smida4, H Chouaieb4, M Chatti4, S Ismail4, R Boukadida1, F Sabri 1, S Ben Amor1, D Zellama1, S Hmissa3, H Kochtali2, A Fathallah4*, Y Guedri1, A Achour1
2022
19
-
v
M Smida1, A Meherzi2, H Chouaieb1, M Chatti1, M Ben Seif1, R Hajlaoui1, M Abdelkefi2, A Fathallah1*
2022
18
-
v
M Sahu1*, M Shah2, MV Rao3, VR Kola4, HK Boorugu5, AR Punjani6, RV Kumar7, S Kumar8, M Manusrut9, RK Rathod10, HK Gonuguntla11, GK Yedlapati12, GR Mallu13, YS Reddy14, RN KomalKumar15, GS Jaishetwar16, KR Balasubramoniam17, SCR Kumar18, B Nagaraju19, PR Sahoo20
2022
17
-
Chronic pulmonary aspergillosis among presumed tuberculosis patients at a tertiary hospital in Ghana
v
BK Ocansey1*, A Adjei2, H Gbadamosi3, C Kosmidis1,4, J Afriyie-Mensah2,5, JO Opintan6, DW Denning1
2022
16
-
v
CD de La Porte1*, CP Provost2, AS Serris1, AC Coste3, MB Bougnoux4, RB Herbrecht5, VB Bru6, FA Ader7, FP Persat8, EC Canet9, FM Morio10, BD Denis11, AA Alanio12, LL Lelièvre13, SC Cassaing14, RS Sonneville15, LM Millon16, OL Lortholary1, ON Naggara2, FL Lanternier1, French Cerebral Mucormycosis Study Group
2022
14
