Date: 26 November 2013
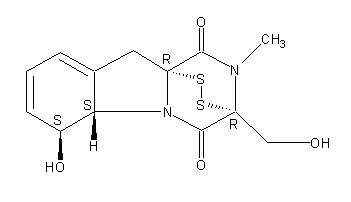
Secondary metabolites, structure diagram: Trivial name – gliotoxin
Copyright: n/a
Notes:
Species: A. flavus, A. fumigatus, A. niger, A. terreus, Eurotium chevalieri, Neosartorya pseudofischeriSystematic name: 10H-3,10a-Epidithiopyrazino[1,2-a]indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-, (3R,5aS,6S,10aR)-Molecular formulae: C13H14N2O4S2Molecular weight: 326.393Chemical abstracts number: 67-99-2Selected references: Larsen TO, Smedsgaard J, Nielsen KF, Hansen MA, Samson RA, Frisvad JC. Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med Mycol. 2007 May;45(3):225-32. Belkacemi, L.; Barton, R. C.; Hopwood, V.; Evans, E. G. V. (CORPORATE SOURCE PHLS Mycology Reference Laboratory, Department of Microbiology, University of Leeds, Leeds, UK). SOURCE Med. Mycol., 37(4), 227-233 (English) 1999 Blackwell Science Ltd. Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP.J Clin Microbiol. 2005 Dec;43(12):6120-2. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center.Toxicity: Gliotoxin posseses a spectrum of biological activities including antibacterial and antiviral activities, and it is also a potent immunomodulating agent. Gliotoxin is also an inducer of apoptotic cell death in a number of cell types, and it has been found to be associated with some diseases attributed directly or indirectly to fungal infections. It is a secondary metabolite produced by a number of Aspergillus and Penicillium species.It is a potent immunosuppressive metabolite and brings about apoptosis in cells. Because of its effects on the immune system it may have a place in transplant surgery. There is limited evidence for its occurrence in moulded cereals. A. fumigatus is a potent pathogen which can colonise the lungs and other body tissues after ingestion of spores. There is some limited evidence that gliotoxin may be formed in situ in such circumstances. hamster LDLo oral 25mg/kg (25mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.mouse LD50 intraperitoneal 32mg/kg (32mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 intravenous 7800ug/kg (7.8mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 oral 67mg/kg (67mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 subcutaneous 25mg/kg (25mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. rabbit LDLo intravenous 45mg/kg (45mg/kg) VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION. GASTROINTESTINAL: HYPERMOTILITY, DIARRHEA Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943. rat LDLo intravenous 45mg/kg (45mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.rat LDLo unreported 50mg/kg (50mg/kg) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943.
Images library
-
Title
Legend
-
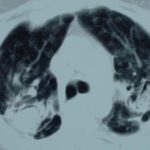
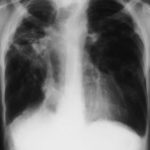
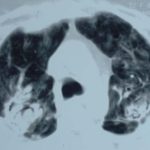
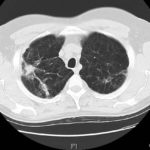
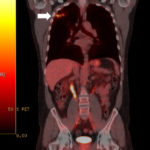
This 54 year old man developed right upper lobe shadowing after development of cough, chest pain and shortness of breath. He suffered from emphysema. Investigations, including a PET scan suggested carcinoma of the lung. He underwent an apical resection and histology showed bullae in the lung with one containing necrotic material and conidial heads consistent with Aspergillus.
Histology:
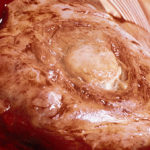
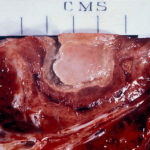
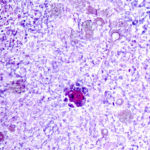
Two wedges of lung tissue were examined. The larger 90 x 35 x 35mm with adherent parietal pleura 100 x 45mm. Sectioning reveals irregular scarring and bullae in the lung tissue. No definite tumour is identified. A focus of possible necrosis up to 10mm in diameter is present, 10mm from the pleura and 20mm from the nearest staple line. Lung and adherent parietal pleura show dense fibrosis around a cavity (see fig A) arrow, lined by granulation tissue and fibrin. This is filled with necrotic material (Fig B) and branching septate fungal hyphae with conidial heads, consistent with Aspergillus and a fungal ball.(Fig C at higher power). A few scattered granulomata are seen away from the cavity. No invasion of lung parenchyma was seen. There is congestion of the lung parenchyma and collections of pigmented macrophages are seen within air spaces.Legends:
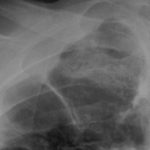
A- Section of lung showing a fungal ball within a cavity (x25). B- Showing branching septate hyphae with conidial heads (x250) C- Higher power magnification (x 500) showing the conidial heads more clearly. D – Edge of cavity showing from left to right – necrosis, granulaomatous reaction, fibrosis, chronic inflammation (x100). E- CT scan (July 08) showing a speculated nodular lesion in the right apex. F – Chest X-ray (May 08) – showing right upper lobe shadowing. G.PET scan (July 08)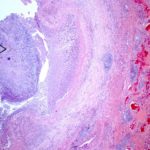 ,
,  ,
,  ,
,  ,
,  ,
,  ,
, 
-
Pt DB This patient with longstanding pulmonary disease developed Mycobacterium avium pulmonary infection which was treated. X rays in Jan 05 through to May 06 revealed an aspergilloma in upper right lobe cavity. Following a severe prolonged bout of coughing up black material- X rays on 22/9/07 showed a disappearance of the aspergilloma.( Images G & H). Go to case history
 ,
,  ,
, 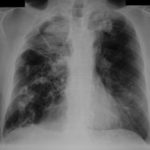 ,
, 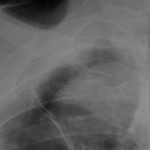 ,
, 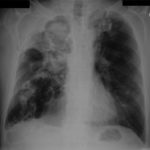 ,
, 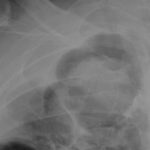 ,
,  ,
, 
-
This 63 year old smoker presented with a new small mass in the right upper lobe. She had had tuberculosis as a teenager (1958) which recurred in 1962, requiring 2 long stays in a sanatorium. Since then she was well, until a new shadow was noticed on her chest X-ray. A CT showed a smooth round nodule, and to rule out carcinoma it was biopsied percutaneously. Histology showed fungal hyphae, consistent with Aspergillus , and serology confirmed infection with Aspergillus fumigatus. Following biopsy, an air fluid pocket has appeared, most consistent with an aspergilloma, as the lesion is solitary.
-
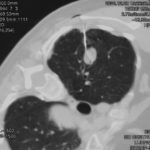
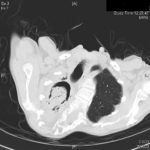
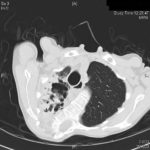
Patient MD with kyphoscoliosis and chronic cavitary pulmonary aspergillosis and an aspergilloma. Patient exhibited azole resistant A. fumigatus.
Further details
Image A. This CT scan cut shown shows a grossly distorted thorax because of the kyphoscoliosis, a nearly normal appearing left lung, her trachea at an odd angle, demonstrating the normal cartilage rings and an aspergilloma in a cavity which has replaced the right upper lobe. The cavity is surrounded by significant pleural thickening and fibrosis.
Image B. The other cut (slightly inferior) shows a complex large cavity and some smaller ones posteriorly, with some material consistent with a fungal ball within the large cavity. There is a separate cavity anteriorly and small air spaces within the extensive pleural thickening. Her trachea is widened. the left lung appears normal.
This patient with repaired juvenile scoliosis first recognised that she had pulmonary aspergillosis when she coughed up large amounts of blood, she was admitted to ICU and underwent bronchial artery embolisation, followed by tranexamic acid orally. A. fumigatus was cultured from sputum. A diagnosis of chronic cavitary pulmonary aspergillosis with an aspergilloma was made. She didn’t improve with itraconazole (no fall in Aspergillus precipitins and continuing symptoms, despite good blood levels) and was treated with voriconazole. She had a good sympomatic response, with marginal improvement in her Aspergillus precipitins titre. Remission continued for over 3 years but then her symptoms of cough and general fatigue returned. Her sputum grew A. fumigatus again, which had MICs to itraconazole (>8 mg/L, resistant), voriconazole (8mg/L, resistant) and posaconazole (2mg/mL, resistant). She is being treated with amphotericin B.
 ,
, 
-
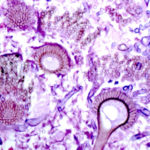
Histopathological appearance of a fungus ball: it consists of a tangled mass of branching, septate hyphal with vesicular swellings in the center (H&E, x 200).

-
Further image details
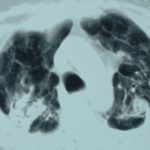
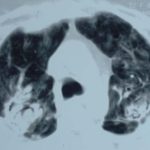
Image A. Long standing sarcoidosis, on corticosteroids with fibrosis and cavitary disease, and a possible fungal ball in the cavity on the left (1996).
Image B. Long standing sarcoidosis, on corticosteroids with 2 cavities containing aspergillomas, one on the left and one on the right (1996).
Image C. Sarcoidosis with progressive cavity formation and aspergillomas. Probable CIPA given appearances (2000).
Image D. Sarcoidosis with progressive cavity formation and aspergillomas. Probable CIPA given appearances (2000).
Image E. Sarcoidosis with progressive cavity formation and aspergillomas. Probable CIPA given appearances (2000).
 ,
,  ,
,  ,
,  ,
,