Date: 26 November 2013
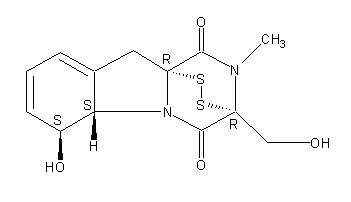
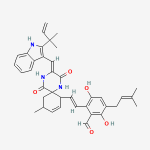
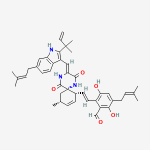
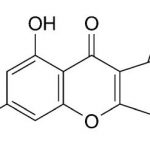
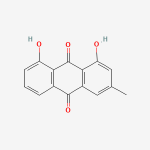
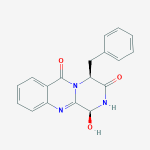
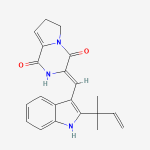
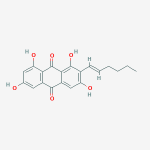
Secondary metabolites, structure diagram: Trivial name – gliotoxin
Copyright: n/a
Notes:
Species: A. flavus, A. fumigatus, A. niger, A. terreus, Eurotium chevalieri, Neosartorya pseudofischeriSystematic name: 10H-3,10a-Epidithiopyrazino[1,2-a]indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-, (3R,5aS,6S,10aR)-Molecular formulae: C13H14N2O4S2Molecular weight: 326.393Chemical abstracts number: 67-99-2Selected references: Larsen TO, Smedsgaard J, Nielsen KF, Hansen MA, Samson RA, Frisvad JC. Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med Mycol. 2007 May;45(3):225-32. Belkacemi, L.; Barton, R. C.; Hopwood, V.; Evans, E. G. V. (CORPORATE SOURCE PHLS Mycology Reference Laboratory, Department of Microbiology, University of Leeds, Leeds, UK). SOURCE Med. Mycol., 37(4), 227-233 (English) 1999 Blackwell Science Ltd. Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP.J Clin Microbiol. 2005 Dec;43(12):6120-2. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center.Toxicity: Gliotoxin posseses a spectrum of biological activities including antibacterial and antiviral activities, and it is also a potent immunomodulating agent. Gliotoxin is also an inducer of apoptotic cell death in a number of cell types, and it has been found to be associated with some diseases attributed directly or indirectly to fungal infections. It is a secondary metabolite produced by a number of Aspergillus and Penicillium species.It is a potent immunosuppressive metabolite and brings about apoptosis in cells. Because of its effects on the immune system it may have a place in transplant surgery. There is limited evidence for its occurrence in moulded cereals. A. fumigatus is a potent pathogen which can colonise the lungs and other body tissues after ingestion of spores. There is some limited evidence that gliotoxin may be formed in situ in such circumstances. hamster LDLo oral 25mg/kg (25mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.mouse LD50 intraperitoneal 32mg/kg (32mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 intravenous 7800ug/kg (7.8mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 oral 67mg/kg (67mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. mouse LD50 subcutaneous 25mg/kg (25mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965. rabbit LDLo intravenous 45mg/kg (45mg/kg) VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION. GASTROINTESTINAL: HYPERMOTILITY, DIARRHEA Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943. rat LDLo intravenous 45mg/kg (45mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.rat LDLo unreported 50mg/kg (50mg/kg) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943.
Images library
-
Title
Legend