Date: 26 November 2013
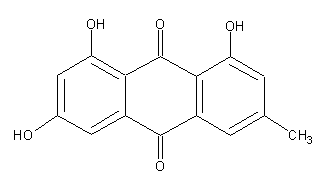
Secondary metabolites, structure diagram: Trivial name – Emodin/Archin/Emodol/Frandulic Acid
Copyright: n/a
Notes:
Species: A. aureus, A. sclerotiorum, A. terreus, A. wentiiSystematic name: 9,10-Anthracenedione, 1,3,8-trihydroxy-6-methyl- (9CI)Molecular formulae: C15H10O5Molecular weight: 270.237Chemical abstracts number: 518-82-1Selected references: Kiriyama, Noriki; Nitta, Keiichi; Sakaguchi, Yoshiaki; Taguchi, Yasuhisa; Yamamoto, Yuzuru (Fac. Pharm. Sci., Kanazawa Univ., Kanazawa, Japan). Chem. Pharm. Bull., 25(10), 2593-601 (English) 1977.Toxicity: Mean lethal dose of emodin orally administered to 1-day-old DeKalb cockerels was 3.7 mg/kg.BACKGROUND: Emodin, a widely available herbal remedy, was evaluated for potential effects on pregnancy outcome. METHODS: Emodin was administered in feed to timed-mated Sprague-Dawley (CD) rats (0, 425, 850, and 1700 ppm; gestational day [GD] 6-20), and Swiss Albino (CD-1) mice (0, 600, 2500 or 6000 ppm; GD 6-17). Ingested dose was 0, 31, 57, and approximately 80-144 mg emodin/kg/day (rats) and 0, 94, 391, and 1005 mg emodin/kg/day (mice). Timed-mated animals (23-25/group) were monitored for body weight, feed/water consumption, and clinical signs. At termination (rats: GD 20; mice: GD 17), confirmed pregnant dams (21-25/group) were evaluated for clinical signs: body, liver, kidney, and gravid uterine weights, uterine contents, and number of corpora lutea. Fetuses were weighed, sexed, and examined for external, visceral, and skeletal malformations/variations. RESULTS: There were no maternal deaths. In rats, maternal body weight, weight gain during treatment, and corrected weight gain exhibited a decreasing trend. Maternal body weight gain during treatment was significantly reduced at the high dose. In mice, maternal body weight and weight gain was decreased at the high dose. CONCLUSIONS: Prenatal mortality, live litter size, fetal sex ratio, and morphological development were unaffected in both rats and mice. At the high dose, rat average fetal body weight per litter was unaffected, but was significantly reduced in mice. The rat maternal lowest observed adverse effect level (LOAEL) was 1700 ppm; the no observed adverse effect level (NOAEL) was 850 ppm. The rat developmental toxicity NOAEL was > or =1700 ppm. A LOAEL was not established. In mice, the maternal toxicity LOAEL was 6000 ppm and the NOAEL was 2500 ppm. The developmental toxicity LOAEL was 6000 ppm (reduced fetal body weight) and the NOAEL was 2500 ppm. Copyright 2004 Wiley-Liss, Inc.
Images library
-
Title
Legend
-
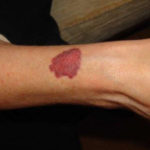
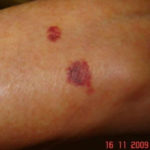
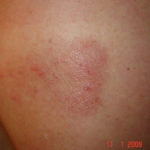
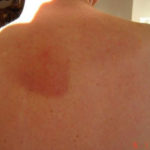
Drug rashes: Drug interactions between steroids and anti-fungal drugs – (ecchymosis)
 ,
,  ,
,  ,
, 
-
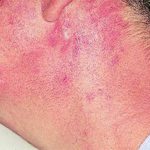
Reference: Muco-cutaneous retinoid effects and facial erythema related to the novel triazole antifungal agent voriconazole. Denning, DW & Griffiths, CEM. Clin.Exp Dermatol 2001, 26(8), 648-53.
Courtesy of Dr D Denning, Wythenshawe Hospital, Manchester.(© Fungal Research Trust)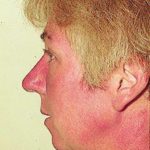 ,
, 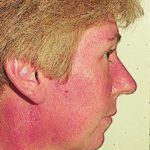 ,
, 
-
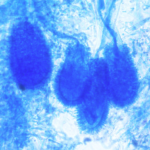
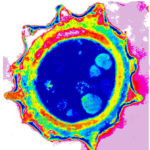
Micrographs of A. niger conidia & conidial heads provided by Amaliya Stepanova, Head of Laboratory pathomorphology and cytology at Kashkin Research Institute, Russian Federation.
 ,
, 
-
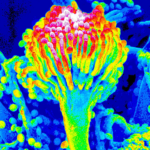
Micrographs of A. terreus conidia & conidial heads provided by Amaliya Stepanova, , Head of Laboratory pathomorphology and cytology at Kashkin Research Institute, Russian Federation.
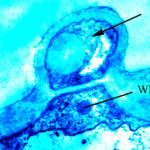 ,
,  ,
, 
-
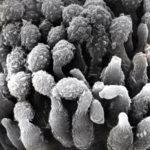
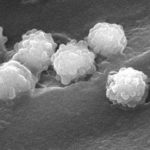
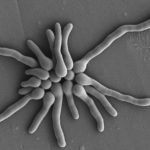
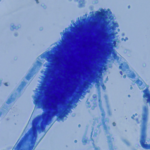
Micrographs of A. fumigatus conidia & conidial heads provided by Amaliya Stepanova, , Head of Laboratory pathomorphology and cytology at Kashkin Research Institute, Russian Federation.
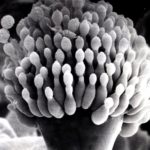 ,
,  ,
,  ,
,  ,
, 
-
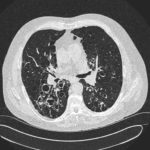
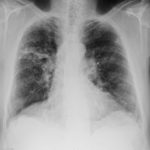
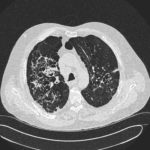
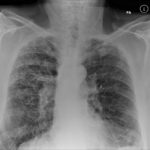
Patients has history of ABPA complicating long standing asthma. His total IgE has fluctuated between 2,200 and 4,600 KU/L, his Aspergillus IgE between 36.3 and 65.4 kAU/L and Aspergillus IgG from 87-154 mg/L. He has been taking long term itraconazole.
 ,
,  ,
,  ,
, 


 ,
,  ,
,