Date: 26 November 2013
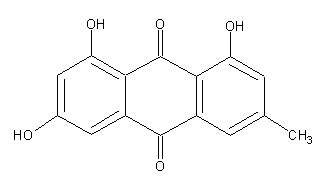
Secondary metabolites, structure diagram: Trivial name – Emodin/Archin/Emodol/Frandulic Acid
Copyright: n/a
Notes:
Species: A. aureus, A. sclerotiorum, A. terreus, A. wentiiSystematic name: 9,10-Anthracenedione, 1,3,8-trihydroxy-6-methyl- (9CI)Molecular formulae: C15H10O5Molecular weight: 270.237Chemical abstracts number: 518-82-1Selected references: Kiriyama, Noriki; Nitta, Keiichi; Sakaguchi, Yoshiaki; Taguchi, Yasuhisa; Yamamoto, Yuzuru (Fac. Pharm. Sci., Kanazawa Univ., Kanazawa, Japan). Chem. Pharm. Bull., 25(10), 2593-601 (English) 1977.Toxicity: Mean lethal dose of emodin orally administered to 1-day-old DeKalb cockerels was 3.7 mg/kg.BACKGROUND: Emodin, a widely available herbal remedy, was evaluated for potential effects on pregnancy outcome. METHODS: Emodin was administered in feed to timed-mated Sprague-Dawley (CD) rats (0, 425, 850, and 1700 ppm; gestational day [GD] 6-20), and Swiss Albino (CD-1) mice (0, 600, 2500 or 6000 ppm; GD 6-17). Ingested dose was 0, 31, 57, and approximately 80-144 mg emodin/kg/day (rats) and 0, 94, 391, and 1005 mg emodin/kg/day (mice). Timed-mated animals (23-25/group) were monitored for body weight, feed/water consumption, and clinical signs. At termination (rats: GD 20; mice: GD 17), confirmed pregnant dams (21-25/group) were evaluated for clinical signs: body, liver, kidney, and gravid uterine weights, uterine contents, and number of corpora lutea. Fetuses were weighed, sexed, and examined for external, visceral, and skeletal malformations/variations. RESULTS: There were no maternal deaths. In rats, maternal body weight, weight gain during treatment, and corrected weight gain exhibited a decreasing trend. Maternal body weight gain during treatment was significantly reduced at the high dose. In mice, maternal body weight and weight gain was decreased at the high dose. CONCLUSIONS: Prenatal mortality, live litter size, fetal sex ratio, and morphological development were unaffected in both rats and mice. At the high dose, rat average fetal body weight per litter was unaffected, but was significantly reduced in mice. The rat maternal lowest observed adverse effect level (LOAEL) was 1700 ppm; the no observed adverse effect level (NOAEL) was 850 ppm. The rat developmental toxicity NOAEL was > or =1700 ppm. A LOAEL was not established. In mice, the maternal toxicity LOAEL was 6000 ppm and the NOAEL was 2500 ppm. The developmental toxicity LOAEL was 6000 ppm (reduced fetal body weight) and the NOAEL was 2500 ppm. Copyright 2004 Wiley-Liss, Inc.
Images library
-
Title
Legend
-
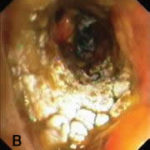
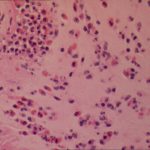
Bronchoscopic manifestations of Aspergillus tracheobronchitis. (a) Type I. Inflammatory infiltration, mucosa hyperaemia and plaques of pseudomembrane formation in the lumen without obvious airway occlusion. (b) Type II. Deep ulceration of the bronchial wall. (c) Type III. Significant airway occlusion by thick mucous plugs full of Aspergillus without definite deeper tissue invasion. (d) Type IV. Extensive tissue necrosis and pseudomembrane formation in the lumen with airway structures and severe airway occlusion (Wu 2010).
-
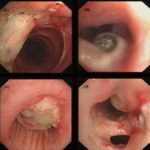
High resolution CT showing centrilobular nodular opacities and branching linear opacities (tree-in-bud appearance) (Al-Alawi 2007).
-
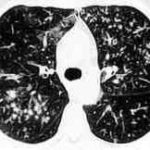
Chest X-ray showing poorly defined bilateral nodular opacities (Al-Alawi 2007).
-
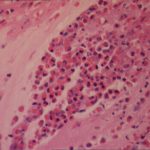
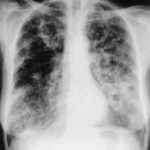
Gross pathologic specimen from autopsy shows the bronchial lumen covered by multiple whitish endobronchial nodules (arrows) (Franquet 2002).

-
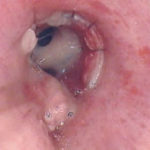
Invasive tracheobronchitis showing numerous nodules seen during bronchoscopy (Ronan D’Driscoll).

-
Pseudomembranous seen overlying the bronchial mucosa (Tasci 2006).