Date: 26 November 2013
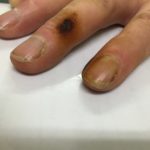
Facial erythema: Voriconazole rash in ABPA patient resistant to corticosteroids, treated with voriconazole 200mg BID. Serum voriconazole levels were very low and the dose was raised to 250mg BID. Within 3 weeks patient had developed remarkable facial erythema. His trough voriconazole concentration at this time was 370ng/ml. When voriconazole was stopped because of the facial erythema and lack of impact on his ABPA his facial erythema resolved over 4 weeks.
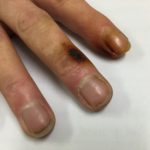
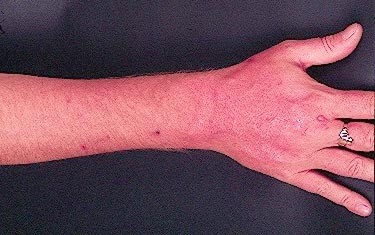
Forearm erythema related to voriconazole. As with facial erythema patient developed remarkable forearm erythema with lesions similar to porphyria cutanea tarda all of which resolved with stopping voriconazole.
Copyright: n/a
Notes:
Images library
-
Title
Legend
-
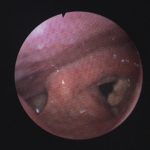
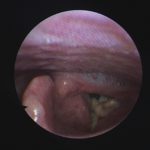
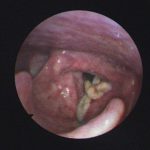
Patient with chronic productive cough, chest pain and ABPA, unable to take itraconazole or nebulised amphotericin B. Smokes at least 40 roll up cigarettes a day.
 ,
, 
-
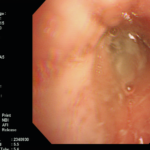
Laryngeal aspergillosis, probably related to inhaled corticosteroids.
 ,
,  ,
,  ,
, 
-
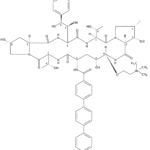
VL-2397 (formerly known as ASP2397) is a novel antifungal drug initially developed by our partner, Astellas Pharma. This drug was isolated from a leaf litter fungus Acremonium species collected in a Malaysian national park. Astellas presented two posters at the 2014 ICAAC meeting which described the in vitro and the in vivo antifungal activities of this drug. The differentiating attributes from the preclinical data of VL-2397 include:
- A novel mechanism of action, with a potential to be complementary or synergistic with the existing classes of antifungals.
- Rapid fungal cell kill activity demonstrated in preclinical models, which was faster than marketed antifungals.
- Activity against azole-resistant fungal species.
- Low propensity for P450 drug-drug interactions.
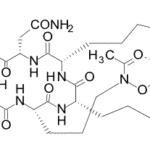
-
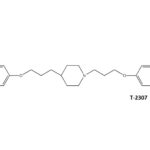
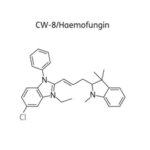
SCY-078, new orally available beta-1,3-d-glucan synthase inhibitor, Formely MK-3118.
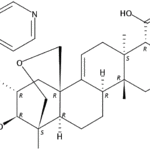
-
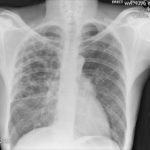
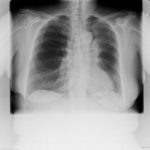
Pt DSM Community acquired primary Aspergillus pneumonia. Two x-rays taken on 02/02/2010 then 05/03/2010
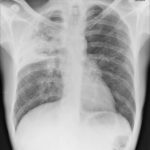 ,
, 



 ,
,