ID 2: 486
Toxin: y
Trivial name:
Emestrin; 2H,8H,20H,22H-1,21-Epidithio-21,1-(iminomethano)-9,13:15,19-dimetheno-4,7,14-trioxa-22a-azacyclononadec[cd]azulene-8,22,26-trione, 6a,22b-dihydro-2,16,20-trihydroxy-12-methoxy-25-methyl-, [1R-(1R*,2R*,6aS*,20S*,21R*,22bS*)]-; Emestrine; [1R-(1R*,2R*,6aS*,20S*,21R*,22bS*)]-6a,22b-Dihydro-2,16,20-trihydroxy-12-methoxy-25-methyl-2H,8H,20H,22H-1,21-epidithio-21,1-(iminomethano)-9,13:15,19-dimetheno-4,7,14-trioxa-22a-azacyclononadec[cd]azulene-8,22,26-trione
Systematic name:
2H,8H,20H,22H-1,21-Epidithio-21,1-(iminomethano)-9,13:15,19-dimetheno-4,7,14-trioxa-22a-azacyclononadec[cd]azulene-8,22,26-trione, 6a,22b-dihydro-2,16,20-trihydroxy-12-methoxy-25-methyl-, (1R,2R,6aS,20S,21R,22bS)-
Molecular formulae:
C27H22N2O10S2
Molecular weight: 598.60
Chemical abstract number: 97816-62-1
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Toxicity:
The effects of emestrin (EMS), a secondary metabolite of the Emericella species, on male ICR mice were examined. The intraperitoneal LD50 values of EMS were 17.7 and 13.0 mg/kg at 24 and 48 hr, respectively. The target organs of EMS were the heart, liver and thymus. In doses over 30 mg/kg the experimental animals died from cardiac failure shortly after the injections. Several survivors that were given EMS in doses under 20 mg/kg showed severe centrilobular necrosis in the liver at 24 hr. Marked degeneration of mitochondria was seen in electron micrographs of both cardiac muscle cells and hepatocytes. In the degenerated hepatocytes, prominent proliferation of RER, membrane-limited inclusions containing both ribosome-like granules and RER, and fenestrated lamella-like structures were observed. Massive necrosis of lymphocytes was always observed in the cortical layer of the thymus of the survivors within 24 hr, while bilateral adrenalectomized mice showed no discernible pathomorphological changes in the lymphoid tissues. Pretreatment of mice with diethyl maleate increased the incidence and severity of hepatic necrosis, whereas that with either cysteine or CoCl2 reduced the severity of centrilobular necrosis of the liver. Pretreatment with phenobarbital had no significant effect on EMS-induced hepatic lesions.
Structure image:
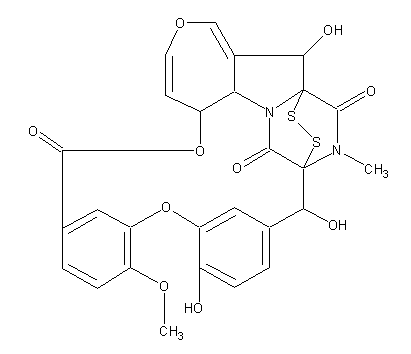
Date uploaded: 2005-12-13 00:00:00
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
