 Aspergillus species are an important cause of life-threatening infections in immunocompromised patients. This population includes patients with prolonged neutropenia, which is the classic risk factor for invasive aspergillosis (IA) including invasive pulmonary aspergillosis (IPA) and is directly related to the degree and the duration of neutropenia (Gerson 1984).
Aspergillus species are an important cause of life-threatening infections in immunocompromised patients. This population includes patients with prolonged neutropenia, which is the classic risk factor for invasive aspergillosis (IA) including invasive pulmonary aspergillosis (IPA) and is directly related to the degree and the duration of neutropenia (Gerson 1984).
Nevertheless, risk stratification of these patients should also include assessment of other factors, including the innate immune system (ie mannose binding lectin) (Lambourne, 2009), other comorbidities e.g. diabetes cardiac failure, cirrhosis, medications such as corticosteroids (O’Donnell 1994, Marr 2002, Guinea 2010) and environmental factors that may precipitate as a whole the occurrence of IA (Nucci, 2014) (Lortholary, 2011; Herbrecht, 2012). Furthermore, a growing number of defined single nucleotide polymorphisms linked to genes affecting the innate immune response have been described which genetically determine susceptibility to IA. Such polymorphisms together with other biomarkers may play a role in the prediction, diagnosis, and management of severe fungal infections in high-risk patients in the coming years (Ok, 2011; Lamoth, 2011; Cunha, 2013; Wojtowicz, 2015).
In the neutropenic host, angioinvasion in IA contributes to the higher frequency of dissemination to other organs such as skin and brain (Hope, 2005; Kosmidis, 2015). IA carries an overall 50% mortality rate even when diagnosed and treated (Garcia-Vidal, 2015) but if the diagnosis is missed or delayed, mortality rate is higher than 95% (Denning, 1996; Brown, 2012).
Prophylaxis
Antifungal prophylaxis should not be used routinely simply because patients have neutropenia. The rationale for antifungal prophylaxis is to prevent fungal infections in a targeted group of high-risk patients for IA, e.g. in severe, prolonged neutropenia (≤ 0.5 x 109 cells/l for >10 days) following myelosuppressive chemotherapy or hematopoietic stem cell transplantation (HSCT) and in those with profound aplastic anaemia.
Fluconazole has a selective action on yeasts so its prophylactic use has no impact on the incidence of fungal infections caused by filamentous fungi such as Aspergillus spp. Intravenous liposomal amphotericin B given in different doses and regimens may have some utility in prophylaxis in high risk patients but its use has been hindered due to its toxicity (Penack, 2006; Mehta, 2006). Aerosolised amphotericin B has proved to be useful clinically when used as prophylaxis with different administration systems, formulations, doses and frequency (Leather, 2006; Rjinders, 2008; Xia, 2015). Comparative randomized trials with azoles are lacking.
Other agents such as itraconazole, voriconazole, posaconazole and micafungin are all proven alternatives as prophylaxis (Zabalza, 2013; Pagano, 2014; Paola, 2014) (Alison, 2011; Ruiz Camps, 2011; Lindsey, 2012). Their major drawbacks are the potential interactions with certain chemotherapy agents, especially cyclophosphamide and vincristine (A quick check can be made here: http://www.aspergillus.org.uk /content/antifungal-drug-interactions), their side effects, especially common with itraconazole solution (gastrointestinal intolerance, hepatic toxicity, heart failure) and voriconazole (photosensitivity or other visual symptoms, hepatic toxicity and gastrointestinal intolerance), and their pharmacokinetics (some of them need fasted conditions to be correctly absorbed e.g. voriconazole and itraconazole solution). Posaconazole has demonstrated high efficacy and a good safety profile in prophylaxis of high-risk haematological patients (Halpern, 2015) and is the antifungal of choice for prophylaxis recommended in infectious diseases guidelines (Cornely, 2007a; Walsh, 2008; NCCN Guidelines, 2011). The new solid oral formulation (tablet) of posaconazole substantially improves bioavailability (Krishna, 2012). Micafungin has also been studied for prophylactic antifungal therapy; however the studies have not achieved sufficient levels of evidence for this strategy, especially in the prevention of IA (Burik, 2004; Huang, 2012).
High risk patients with neutropenia should receive prophylaxis until their neutrophils levels are above 1000/µl (Ruiz Camps, 2010).
Serological monitoring for invasive aspergillosis
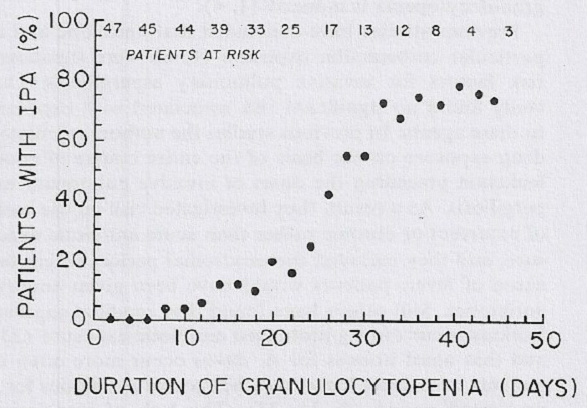
Early diagnosis of IA is usually difficult as most signs are absent or nonspecific in the absence of neutrophils. The rate of decline of the neutrophil count and the duration of neutropenia are critical factors. These two aspects are a measure of bone marrow reserve and are highly correlated with severity of infection and clinical outcome (Lindsey, 2012).
Response to treatment and survival can, potentially, be improved if specific antifungal treatment for IA is initiated at an early stage of infection (Morrisey, 2014). However, an early diagnosis of IA based on histopathological or mycological evidence can be difficult to establish. Microscopic examination, culture and identification of fungi in samples, despite being the gold-standard technique for diagnosis of IA, have very low sensitivity (<50%) and they are usually positive when the fungal burden is high and the infection is at an advanced stage (Brown, 2012).
Detection of galactomannan (GM), an Aspergillus cell-wall component released during fungal growth, in serum and bronchoalveolar lavage (BAL) fluid by enzyme-linked immunoassay (ELISA) improves early detection of aspergillosis and complements CT scans. Large meta-analyses have shown a sensitivity and specificity of the test in high-risk haematology patients of about 70% and 90% respectively (Pfeiffer, 2006; Leeflang, 2008; Zou, 2012; Heng, 2013). GM has a high negative predictive value, being a useful technique as a screening tool to exclude IA, especially in those not on prophylaxis (Pereira, 2005). Studies have reported that GM can be detected a median of 5-8 days before culture positivity (Miceli, 2008) and serial GM monitoring has been shown to significantly shorten the time of diagnosis of IA in high-risk patients with onco-haematological diseases. However, several variables including antifungal therapy or prophylaxis significantly reduce levels of circulating galactomannan, reducing sensitivity by 30% (Cuenca-Estrella, 2011; Duarte, 2014). Some authors have suggested reducing GM cut-off value to 0.5 to increase sensitivity, especially in high risk patients receiving prophylaxis (Marr, 2004). False-positive results have been reported in several contexts, including in patients who have received certain antibiotics (Beta-lactam antibiotics, especially piperacillin-tazobactam), and patients with other invasive mycoses (including Penicillium infection, histoplasmosis, and blastomycosis).
Beta-D-glucan is also a component of the fungal cell wall, but it is not specific for Aspergillus since it is present in many fungi (Candida, Fusarium, Trichosporon and Pneumocystis species) (Verweij, 2006). In a recent meta-analysis, the pooled sensitivity and specificity for Beta-D-glucan among patients with invasive fungal infections was 75.6% and 85.3%, respectively (Karageorgopoulos, 2011). There are not enough data published on IA detection during profound neutropenia to be confident of its utility in this setting. False-positive results are more common in patients on dialysis or those who are infected with certain Gram-negative bacteria, such as Pseudomonas aeruginosa. The test has been incorporated into some guidelines as a criterion for a probable invasive fungal infection (Yoshida, 2006; De Pauw, 2008).
Polymerase chain reaction (PCR) detection of Aspergillus DNA in serum, BAL fluid or respiratory tract biopsy has a potential for rapid and early detection of IA (Thomas, 2013; Melinda, 2015; White, 2015a). The greatest limitation of PCR has been the lack of standardization and validation, the reason why it was excluded in 2008 from the European Organisation for Research and Treatment of Cancer/Mycoses Study group (EORTC/MSG) definitions of invasive fungal disease (De Pauw, 2008; Kourkoumpetis, 2012). Since 2006, several PCR tests have been commercialised and the European Aspergillus PCR Initiative (EAPCRI), which involves centres across Europe, Australia and the Middle East, have been looking for a standard Aspergillus PCR methodology so that PCR could be incorporated into future consensus definitions for invasive fungal disease. A meta-analysis of clinical trials that evaluate the accuracy of the PCR assay for IA concluded that the presence of at least two positive whole-blood PCR specimens in a high-risk patient should be considered very indicative, if not confirmatory, of IA, with a specificity of 95% and a sensitivity of 64% (Arvanitis, 2014; White, 2015b). Currently, PCR is the only nonculture assay that has the capability of being genus and species specific, and has the added potential to determine azole resistance. Monitoring the Aspergillus DNAemia and serum GM have shown to provide better guidance for antifungal treatment by identifying earlier subclinical stages of infection in high-risk patients (Morrissey 2013; Arvanitis, 2015; Aguado 2015).
Radiological monitoring for invasive aspergillosis
Chest radiographs are normal in about 10% of patients with ultimately fatal IPA (Denning 1998a) and chest computed tomography (CT) is much more sensitive, but also not perfect (Weisser, 2005). IA in severely immunocompromised patients typically involves the lung, and CT detects lung involvement at an early stage of infection, but often slightly after GM or PCR (Arvanitis, 2015). The combined use of PCR, serum galactomannan and detection of pulmonary infiltrates by early use of CT should improve detection of IPA and permit earlier initiation of antifungal therapy.
High resolution chest CT (HRCT) for the diagnosis of IPA has high sensitivity (89%) and its systematic use is associated with improved outcomes (Caillot, 2001a; Morrisey, 2014). Most patients with IPA have macronodules and many also have the halo sign (Greene 2007; Cuenca-Estrella, 2011; Wingard, 2012). The halo sign represents an earlier stage of IPA and is the radiological representation of lung infarction that follows angioinvasion by hyphae. Its presence in febrile neutropenic patients must be interpreted as suggestive of an invasive mould disease and its identification allows early initiation of antifungal treatment and therefore, improvement of outcome. It should be noted that the halo sign is not specific for invasive aspergillosis or even any invasive fungal disease, as other infections and clinical entities can have similar radiographic patterns. Other findings noted with IA are consolidation, infarct-shaped nodules, cavities and air-crescent signs. A pleural effusion is uncommon, but is seen. Cavitary lesions, including the air crescent sign, are considered to be characteristic of later-stage disease upon recovery of neutrophils (Greene, 2007; Marcio, 2013).
Some studies have demonstrated that the first CT abnormality of IPA is the bronchoalveolar pattern that presents different and usually ‘non-specific’ radiological findings including areas of peribronchial consolidations, micro nodules (≤1cm) centrilobular nodular opacities, ground glass infiltrates, branching linear or nodular opacities with a ‘tree-in-bud’ appearance and focal areas of bronchiectasis. Early diagnosis in this phase would mean prevention of the angioinvasive phase and would contribute to the decrease in mortality. It is important to point out that the more severely neutropenic the host is, the faster and more likely is the angioinvasion, making the early bronchoalveolar phase very short in these patients (Mircescu, 2009; Nucci, 2010).
It is well documented that the infiltrates in patients with IA worsen over the first week of therapy (Caillot 2001b), even though with continued therapy the patients respond. This is related in large part, to the changing immune status of the patient. Neutrophil recovery or withdrawal of immunosuppressive therapy may exacerbate inflammatory responses, leading to larger pulmonary infiltrates, persistent or worsening fever, and clinical manifestations, the so-called immune reconstitution syndrome. Serial GM testing can be helpful in distinguishing those truly not responding and those who are responding in the face of clinical worsening (Miceli, 2008; Wingard, 2008).
One of the particular problems is the management of small nodules, which may or may not represent IPA. A thoughtful approach to this in haematological malignancy was recently published (Wingard 2012).
Criteria for initiation of therapy
The incidence of invasive fungal infection rises after patients have experienced more than seven days of persistent neutropenia. Guidelines recommend treatment in neutropenic patients in the following situations (Aguilar-Guisado, 2010; Alison, 2011; Vallejo, 2012):
- Empirical antifungal treatment after four to seven days of persistent or recurrent fever in spite of broad spectrum antibiotics in high risk neutropenic patients who are expected to have a total duration of neutropenia >7 days. Some authors have shown similar effectiveness following a diagnostic and therapeutic approach based on risk profile and driven by clinical criteria (Maertens, 2005; Aguilar-Guisado, 2012).
- Patients with prolonged neutropenia who have any microbiological, serological and/or radiological sign of IPA, e.g. positive cultures, high levels of GM or suspected images in CT scan (De Pauw, 2008).
- Patients with any clinical features of IA; notably chest pain or discomfort, new cough, haemoptysis, nasal stuffiness or any new facial discomfort or swelling, new localised purple or black skin or oral lesions, a fit, stroke or transients ischaemic attack.
Choice of antifungal
Due to high rates of mortality of IA in immunocompromised patients, prompt initiation of antifungal therapy is crucial. Initiating treatment as diagnostic-driven therapy (based on X-ray findings, GM, beta-D-glucan and PCR) prior to the mycological documentation of the disease was reported to significantly improve the 12-week survival rate (Pagano, 2007; Nivoix, 2008; Herbrecht, 2012; Morrissey, 2014).Treatment with amphotericin B within the first ten days of therapy lead to only 40% mortality, compared to 90% if treatment was started after ten days (von Eiff, 1995).
Aspergillus fumigatus followed by Aspergillus flavus are the most common species recovered from cases of IA (Klingpor, 2015). Aspergillus terreus is observed with high frequency at several centres (Lass-Florl, 2005) and is notable for being intrinsically resistant to amphotericin B. Aspergillus niger is an unusual cause of IA and it appears to be less sensitive than other species to itraconazole (Howard, 2013) and isavuconazole (Espinel-Ingroff, 2013). Aspergillus nidulans also rarely causes IA and it is frequently resistant to amphotericin B (Segal, 1998, Van der Linden, 2011).
The largest prospective randomized trial for the treatment of IA demonstrated that voriconazole was superior to amphotericin B as first line therapy and found to be associated with higher response rates (53% vs. 32%; p < 0.001) and an improved survival rate (71% vs. 58%; p = 0.02) (Herbrecht, 2002; Patterson, 2005). Hence, voriconazole is approved for the primary treatment of invasive aspergillosis in most patients. Intravenous (iv) loading with 2 doses of 6mg/kg followed by 4mg/kg iv twice a day for 7 to 10 days should be given before converting to oral therapy. The oral continuation dose is usually either 200mg or 300mg twice a day (Leather, 2006). Levels should be taken to guide treatment and determine the need for dosage escalation during the oral phase.
For patients for whom voriconazole is contraindicated (notably drug interactions), or in whom it is ineffective or if an azole-resistant strain has been isolated, liposomal amphotericin B (3mg/kg/day) is one alternative (Cornely, 2007b; Denning, 2007). In non-neutropenic patients, the echinocandins may be useful. All three available echinocandins (caspofungin, micafungin and anidulafungin) have activity against Aspergillus spp; Caspofungin is licensed for salvage therapy of IA (Maertens, 2004; Morrisey, 2006; Dockrell, 2008; Hiemenz, 2010) and there are some data supporting its use as primary therapy (Zaoutis, 2009; Viscoli, 2009; Herbrecht, 2010; Raad, 2015). Prospective treatment data also shows a similar level of efficacy for micafungin (150 mg daily) alone or in combination with other systemic antifungal agents (Denning, 2006; Kohno, 2013).
Posaconazole is used as an alternative therapy in patients intolerant or with contraindications to voriconazole and amphotericin B (800mg administered in two or four divided doses as suspension) (Walsh, 2007). There was a strong relationship between response rates and serum posaconazole concentrations, with only a 24% response rate in those with a median steady state concentration of ~140ng/mL and 75% in those with concentrations of 1500ng/ml. The replacement of posaconazole solution with tablets, which have markedly better bioavailability, should improve the overall response rate considerably, and intravenous posaconazole is also available.
Isavuconazole is a new extended-spectrum azole that has been recently approved by the FDA for the treatment of IA; some advantages of this triazole are the availability of a water-soluble intravenous formulation, excellent bioavailability of the oral formulation, predictable pharmacokinetics, and, to date, few adverse effects. A randomized, double-blind clinical trial showed non-inferiority comparing it with voriconazole for primary treatment of IA (Raad, 2014; Miceli, 2015), having been studied specifically, in neutropenic patients (Patterson, 2014). The recommended dosage regimen is a loading dose of 600mg given as 200 mg every 8 hours for 2 days followed by 200 mg daily thereafter.
The addition of a second antifungal agent to a first agent that is failing or toxic is usually practiced out of understandable desperation. Nevertheless, limited non-randomized clinical trial data suggest the benefit of some forms of combination therapy against IA (Maertens, 2006; Singhs, 2006), but there are still insufficient clinical data to support its use as primary treatment, and many retrospective series do not show benefit for combination therapy. A prospective combination study of voriconazole with or without anidulafungin has been published recently (Marr, 2015). Overall, there appeared to be a non-significant benefit with those with moderate GM antigen levels benefitting most, and also non-neutropenic patients. These data are most consistent with a lack of antagonism between voriconazole and an echinocandin, which is a concern for azole amphotericin B combinations, but insufficient to make a general recommendation for combination therapy for invasive aspergillosis (Kontoyiannis, 2003; Marr, 2004; Kontoyiannis, 2005; Cesaro, 2007; Thomas, 2010; Raad, 2015).
Several reports have been published and surveillance studies have been performed showing an increase of azole resistance in A. fumigatus (ECDC, 2013; Van der linden, 2015). If an azole-resistant A. fumigatus strain is isolated, combination and/or substitution of therapy is recommended, as set out by an expert panel recently (Verweij, 2015). In addition, molecular identification of the resistance mechanism should also be performed for epidemiological reasons although this should not delay MIC determination or treatment modification. Azole resistance is commonly due to mutations in the cyp51A-gene, which encodes the target enzyme of antifungal azoles, and A. fumigatus colonies cultured from patients receiving long-term treatment can represent different azole-resistant genotypes. Surveillance studies show that, in areas which Aspergillus is endemic, the environmental route of resistance selection contributes to >90% of resistance mechanisms in azole therapy (Snelders, 2008; Van der linden, 2011a). The prognosis for individuals infected with a resistant Aspergillus strain is poor, with nearly 90% mortality against an expected mortality of ~50% for invasive aspergillosis (Van der Linden, 2011b; Chowdhary, 2013; Denning, 2015).
Neutropenic patients with IA have a slightly risk of co-infection with another mould, the most common of which are the Mucorales (Perkhofer, 2010; Klingspor, 2015;). Because filamentous fungal infections are difficult to diagnose, a progressive pulmonary infiltrate in the setting of proven or probable invasive aspergillosis may be caused by mucormycosis or another unusual mould for which a lipid formulation of amphotericin B may be effective (Auberger, 2012; Thomas, 2013;), although posaconazole and isavuconazole are also partially effective. Prospective studies of this problem are very difficult to undertake, hence the uncertainty about the recommendations.
Data are insufficient to recommend a specific empirical antifungal agent for a patient already receiving antifungal prophylaxis, but switching to a different class of antifungal that is given intravenously should be considered (Alison, 2011; Lerolle, 2014;). Within the triazole class with activity against Aspergillus spp., the structures of itraconazole and posaconazole are most similar and voriconazole and isavuconazole are also similar, and can be thought of as sub-classes (Additional information here: /article_database/antifungal-drug-summaries-product-characteristics-spc). Given this, a switch from one sub-class to another is appropriate if azole fungal resistance is unlikely, as the most common reason for failure is pharmacodynamics (Walsh, 2008; Winston, 2011; Dolton, 2012; Cho, 2015;). For example, 6 of 7 patients who had IA breakthrough on posaconazole, responded to voriconazole (Dolton 2012). There are many other examples of itraconazole prophylaxis breakthrough responding to voriconazole.
Surgery for invasive pulmonary aspergillosis
The combination of antifungal agents and surgical resection could be an efficient strategy for the treatment of IPA in some patients with hematologic malignancy. Apart from a diagnostic open lung tissue biopsy, there are 3 primary indications for surgical interventions in IPA (Bernhard, 2008; Shakil, 2013):
- To arrest severe haemoptysis.
- To prevent severe haemoptysis in patients with centrally located lesions.
- To remove lesions as a component of secondary prophylaxis, prior to additional chemotherapy and/or stem cell transplantation.
In immunocompromised patients with IPA and especially in neutropenic patients, massive haemoptysis directly causes death in 2-4 % of patients in this setting. Cavitary lesions are more susceptible to bleeding. During profound neutropenia, pulmonary foci of disease are a result of direct vascular invasion by the fungus (angioinvasion) which leads to infarction and necrosis. Pulmonary cavitation, and sometimes an air crescent, often becomes visible radiologically. Progressive destruction of lung includes direct invasion of adjacent vascular structures with rupture and bleeding, notably as the neutrophil improves rapidly with an influx of cells into infected areas (Todeschini, 1999).
Surgical intervention in general is indicated in significant haemoptysis (often urgently), even if thrombocytopenia persists (platelet transfusions should be given immediately before surgery) (Wong, 1992; Bernard, 1997; Baron, 1998). Surgery should be combined with antifungal therapy, and enough platelet transfusions to bring the level up to 50 x 109/L.
In those with an IPA lesion close to one or more of the great vessels, resection should be urgently considered as a preventive measure as these lesions carry a high risk of bleeding (Moreau, 1993; Caillot, 2001; Walsh, 2008). Once such a lesion has been identified, surgery should be undertaken as fast as possible. Repair of the pulmonary artery or vein is quite likely to be required during surgery.
In those with persistent lesions on CT scan who require additional myeloablative chemotherapy or stem cell transplantation for control or cure of their leukaemia or other underlying disease, may benefit of surgical resection of one or more lesions (Reichenberger, 2002; Ali, 2006). Sometimes this requires bilateral resections. It is always recommended that antifungal treatment be given pre- and postoperatively until clinical symptoms and radiological signs resolved.
In those with bilateral lesions and a diffuse or multi-focal appearance on the CT scan, the surgical approach is associated with difficult postoperative course and relapse of infection (Danner, 2008).
Secondary prophylaxis
Patients who recover from an episode of IA are at risk for relapse of infection during subsequent immunosuppression, up to 30-50% of patients without adequate prophylaxis. A mould-active agent is recommended in patients with prior invasive aspergillosis, anticipated prolonged neutropenic periods of at least 2 weeks, or a prolonged period of neutropenia immediately prior to HSCT. Secondary prophylaxis is generally administered for the duration of immunosuppression (Alison, 2011; Lindsey, 2012).
Outcome
Despite improvements in the antifungal armamentarium and diagnostic modalities, IA continues to be associated with significant morbidity and mortality in high-risk patients. Identification of some prognostic factors, possibly including genetic risk factors, may help to determine which patients require risk modification and/or more aggressive treatment (Upton, 2007). As we move toward personalized medicine and targeted therapy in oncology, efforts to allow for more individualized choices in fungal therapy are ongoing, with work being done on risk prediction scores for IFIs development, immunogenetic profiling to determine host-specific risk factors for IFI-infection, and individual mycobiome monitoring. Independent prognostic factors include neutropenia, renal insufficiency, hepatic insufficiency, early onset IA, proven IA, methylprednisolone use and early-onset of treatment (Baddley, 2010; Perkhofer, 2010; Ramos, 2011). Overall, the main challenge is to overcome breakthrough fungal infections (Barchiesi, 2015).
Isabel Rodriguez Goncer, MD
Hospital Universitario de Mostoles
Rio Jucar street, 28935
Mostoles (Madrid, Spain)
Email: isargoncer@gmail.com
David W. Denning,
Professor of Medicine and Medical Mycology
Director, National Aspergillosis Centre
Education and Research Centre
University Hospital of South Manchester
Southmoor Road
Manchester M23 9LT, UK
December 2015
