Submitted by Aspergillus Administrator on 18 January 2013
Modern medicines have to be tested on animals to try to ensure that they are safe before they go to being ‘tested’ on people. This has long been the view of various animal rights groups and it has also long been stated by those supporting the use of animals for experimentation that their use cannot be substituted.
The cold facts are that over 300 000 people a year are still killed in the US and Europe despite all the animal testing. Over 90% of drugs that make it through animal testing subsequently fail, many due to toxic effects not identified by animal testing – so this is an expensive problem as well as highly inefficient as a safeguard for human health. There are examples of modern drugs that were so toxic to humans that volunteers were severely injured when testing them for the first time. As a consequence most of these trials are said to have moved to India (where regulation is less strict) and it is reported that thousands of people have died in India since 2006.
We clearly have a moral and financial need to improve the accuracy and predictive efficiency of how we test new drugs for toxicity.
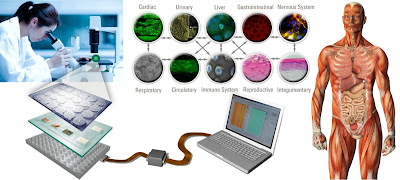 |
| Body on a chip research |
A recent article in New Scientist by the seems to be saying that times are starting to change. There are now some alternatives to the use of animals that are at least partially viable and need further use to develop. Examples include (quoting the NS article)
These techniques include: human tissue created by reprogramming cells from people with the relevant disease (dubbed “patient in a dish”); “body on a chip” devices, where human tissue samples on a silicon chip are linked by a circulating blood substitute; many computer modelling approaches, such as virtual organs, virtual patients and virtual clinical trials; and microdosing studies, where tiny doses of drugs given to volunteers allow scientists to study their metabolism in humans, safely and with unsurpassed accuracy. Then there are the more humble but no less valuable studies in ethically donated “waste” tissue.
Most of these look like they need a lot more development but with ever increasing power given to us to analyse the human body (genome sequencing, expression analysis, Proteome studies) they may no longer be ‘pie in the sky’ dreams. These techniques may start to provide real alternative to some animal testing – possible small scale to begin with but given the rather poor record of animal testing when trying to predict toxicity they may become an additional tool fairly quickly.
One problem is that animal testing is compulsory throughout most of the pharmaceutical development world, so until laws can be changed animals cannot be replaced. At our best guess, this is going to be a gradual process.
News archives
-
Title
Date


