Submitted by GAtherton on 13 October 2017

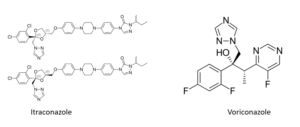
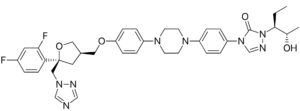
Pulmazole is a preparation of itraconazole dispersed in an inhalation powder. Intended to treat fungal lung infections it has been awarded the designation ‘Qualified Infectious Disease Product’ by the US Food and Drug Administration. This is the second such designation for this preparation, the first being made for the treatment of people with cystic fibrosis who had a fungal lung infection, a common diagnosis for that group affecting around 15% of all cystic fibrosis patients.
The second FDA designation is for the treatment of allergic bronchopulmonary aspergillosis (ABPA) in asthmatic patients. Approximately 2.5% of asthma patients (625 000 people) in the US have ABPA which is a condition caused by Aspergillus fumigatus and which is currently treated with oral itraconazole.
Like most systemic antifungal medication oral itraconazole has a number of unpleasant side effects and interactions with many other medications so its delivery as an inhaled product should reduce those effects while at the same time target the lungs as the main area of activity.
News archives
-
Title
Date


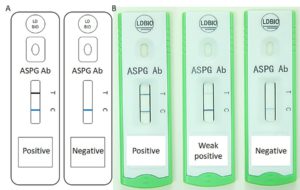



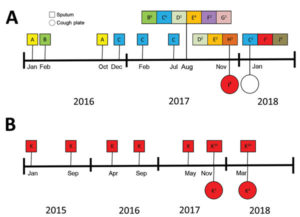
![Gulu referral hospital[2] Gulu referral hospital[2]](https://www.aspergillus.org.uk/wp-content/uploads/2019/03/Gulu-referral-hospital2_0-232x300.png)