Submitted by Aspergillus Administrator on 6 July 2009

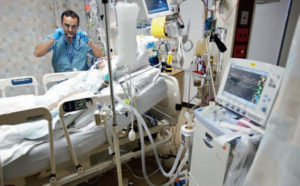
The Food and Drug Administration (FDA) in the United States has issued a safety update for a drug used to treat active rheumatoid arthritis. They now recommend that all patients must be screened for tuberculosis and other pulmonary infections such as aspergillosis prior to taking Leflunomide due to the risk that the drug will increase the chances that these infections will get worse.
Medications with immunosuppressive potential, such as leflunomide, may increase patient susceptibility to opportunistic infections, particularly tuberculosis (including extrapulmonary disease), Pneumocystis jiroveci pneumonia, and aspergillosis.
The FDA notes that leflunomide has not been studied in patients with a positive tuberculin screen result, and the safety of leflunomide in those with latent infection remains unknown. Patients with a positive test result should be treated by standard medical practice before starting leflunomide therapy.
Leflunomide is indicated to reduce signs and symptoms of disease, inhibit structural damage, and improve physical function in patients with active rheumatoid arthritis.
News archives
-
Title
Date

 ,
, 



 ,
,