Submitted by GAtherton on 8 July 2015
EUCAST has played a major role in the standardisation and harmonisation of the protocols & drug levels used to decide if a pathogen is susceptible, intermediate or resistant to antibiotic/antifungal drugs. Assessing the minimal inhibitory concentration (MIC) of an antimicrobial drug for a specific microbial (i.e fungal or bacterial) isolate is vitally important when doctors need to know which drug to give a patient and what dose to
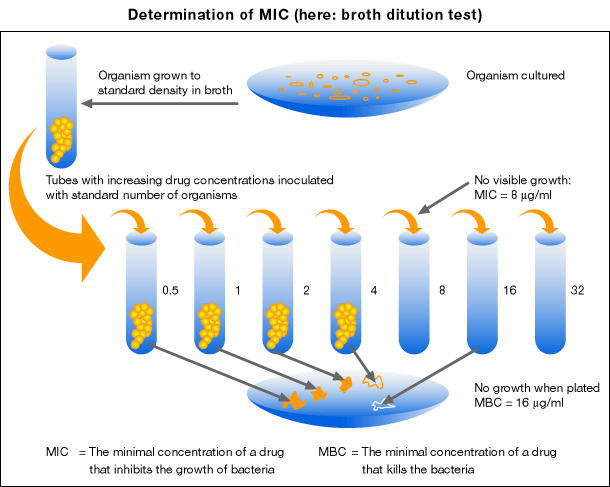
use as to give too little promotes the development of resistance and too much is prone to causing too many side effects for the patient.
Accurate definitions of the breakpoints (specific drug doses defined by MIC tests) used to decide if a pathogen is either susceptible, intermediate or resistant are also common currency amongst doctors and it is important that they are accurate and set according to agreed standards.
Both are also important for research work into resistance.
If one country is not using exactly the same protocols to test for MIC & breakpoints they may get different answers to other countries, confusing all parties and potentially delaying adequate treatment & better research.
EUCAST has ensured that all countries in the EU have worked together to ensure the measurement of MIC & breakpoint is consistent across all EU member countries. It has been a difficult task but there are signs it is nearing completion. The 2014 Garrod Lecture report by Kahlmeter suggests a good degree of harmonisation has been reached and points out that dissemination of all the relevant data is freely available on the EUCAST website, aiding and easing wider use of these new standards.
.
News archives
-
Title
Date


