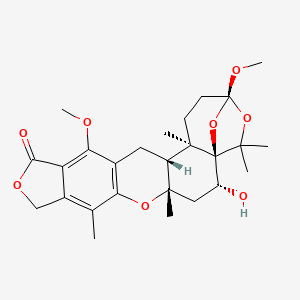
Systematic name:
5H-3,5a-Epoxy-12H-furo[3,4-i]oxepino[4,3-a]xanthen-12-one, 1,2,3,6,7,7a,10,14,14a,14b-decahydro-6-hydroxy-3,13-dimethoxy-5,5,7a,9,14b-pentamethyl-, (3S,5aR,6R,7aS,14aR,14bR)-
Molecular formulae:
C26H34O8
Molecular weight: 474.5
Chemical abstract number: 81543-02-4
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Structure image:
Mycotoxin & Metabolites
Showing 10 posts of 547 posts found.
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
