ID 2: 182
Toxin: n
Trivial name:
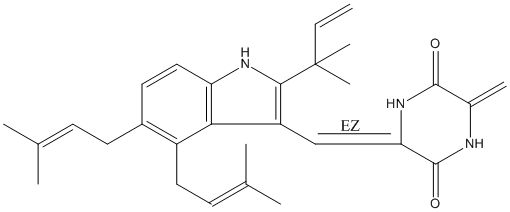
2,5-Piperazinedione, 3-[[2-(1,1-dimethyl-2-propenyl)-4,5-bis(3-methyl-2-butenyl)-1H-indol-3-yl]methylene]-6-methylene-, (3Z)- (9CI); 2,5-Piperazinedione, 3-[[2-(1,1-dimethyl-2-propenyl)-4,5-bis(3-methyl-2-butenyl)-1H-indol-3-yl]methylene]-6-methylene-, (Z)-; Cryptoechinuline G
Systematic name:
2,5-Piperazinedione, 3-[[2-(1,1-dimethyl-2-propenyl)-4,5-bis(3-methyl-2-butenyl)-1H-indol-3-yl]methylene]-6-methylene-, (3Z)-
Molecular formulae:
C29H35N3O2
Molecular weight: 457.6
Chemical abstract number: 68836-03-3
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Toxicity:
Antiinflammatory
Structure image:
Date uploaded: 2008-07-07 15:48:31
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
