ID 2: 377
Toxin: n
Trivial name:
1-Demethoxyreserpine; 11-Desmethoxyreserpine; Canescin; Canescine; Canescine (Rauwolfia); Deserpic acid, methyl ester, 3,4,5-trimethoxybenzoate; Deserpidin; Harmonyl; NSC 72138; Raunormin; Raunormine; Recanescin; Recanescine; Reserpidine
Systematic name:
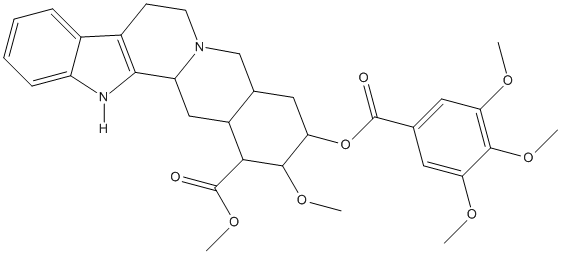
Yohimban-16-carboxylic acid, 17-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-, methyl ester, (3β,16β,17α,18β,20α)-
Molecular formulae:
C32H38N2O8
Molecular weight: 578.7
Chemical abstract number: 131-01-1
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Structure image:
Date uploaded: 2008-07-07 15:48:31
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
